For patients

Chiba University Hospital
Center for Advanced Medicine

Natural Killer T (NKT) cells represent a unique lymphocyte subpopulation. After activation, NKT cells present a strong anti-tumor activity against various head and neck cancers, such as tongue cancer, pharyngeal cancer, and laryngeal cancer as well melanoma in nasal and oral cavities. We have conducted several clinical studies, and have so far found that nasal administration of a small number of dendritic cells from the peripheral blood, pulsed with a specific glycolipid that activates NKT cells, exhibits significant clinical results without any serious side effects. We are planning to conduct a new clinical study to demonstrate the efficacy of this treatment in preventing the recurrence of cancer for patients who have been treated with radiation therapy, chemotherapy, or those who have undergone surgery. If you are interested and would like to know more about this treatment, please contact us below.
Yoshitaka Okamoto, MD, PhD
Email: yokamoto (Here at mark) faculty.chiba-u.jp
Phone: +81-43-226-2289
Naoki Kunii, MD, PhD
Email: kunii (Here at mark) chiba-u.jp
Phone: +81-43-226-2718
Fax: +81-43-226-2718
NKT cells are a unique lymphocyte subpopulation characterized by the expression of both NK receptor and T cell receptors. NKT cells recognize a specific glycolipid antigen, α-Galactosylceramide and show a strong anti-tumor activity against various types of cancer. To apply the potent anti-tumor activity of NKT cells to clinical fields, we have conducted several clinical trials of NKT cell-based immunotherapy in patients with non-small cell lung cancer. As a result, the intravenous administration of α-Galactosylceramide-pulsed dendritic cells exhibited some clinical effects including prolonged overall survival. We are now conducting a phase II clinical trial in patients unresectable advanced or recurrent non-small-cell lung cancer to test the efficacy of the treatment. If you are interested in NKT cell based immunotherapy, please contact us.
Shinichiro Motohashi, MD, PhD
Email: motohashi (Here at mark) faculty.chiba-u.jp
Phone: +81-43-226-2718
Critical limb ischemia and refractory angina are serious symptoms of atherosclerosis and have few therapeutic options. Recent investigations revealed that several types of molecules and cells serve to promote neo-vascularization in these diseases. Attempts to enhance neo-vascularization for these cells are called therapeutic neovascularization.
We found that a certain fraction of blood cells, called mononuclear cells (MNC) have great ability to promote neo-vascularization (Figure1) in ischemic tissues. We have applied MNC therapy for patients with critical limb ischemia and refractory angina. We started our clinical trial in 2002, and so far it is a relatively safe and effective option to treat patients (Figure2).
Nevertheless, we also recognize that, to better serve otherwise patients with no options for treatment we need to elucidate a more detailed mechanism for the development of diseases and its therapeutics. We at the CAM vigorously conduct our research, which reciprocally integrates bench and bedside investigations.
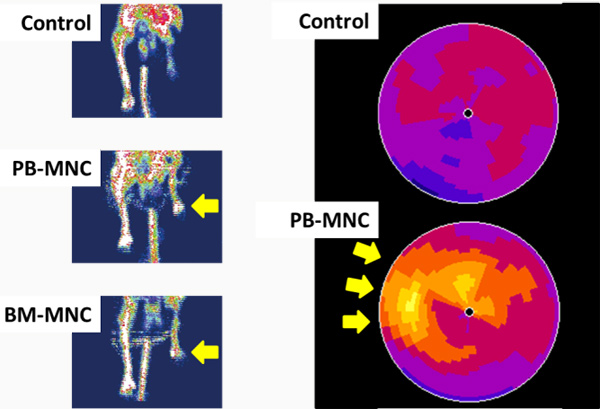
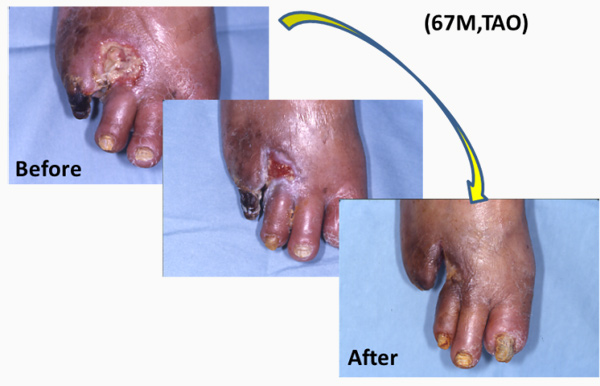
Kaoru Tateno, MD, PhD
Email: tateno.kaoru (Here at mark) gmail.com
Phone: +81-43-226-2555
Lecithin: cholesterol acyltransferase (LCAT) is an enzyme responsible for cholesterol esterification in plasma. LCAT deficiency syndrome is an autosomal recessive disorder with mutations in the LCAT gene characterized by low HDL-cholesterol and corneal opacity. Abnormal lipid metabolism caused by LCAT dysfunction in patients leads to progressive renal insufficiency and end-stage renal failure in severe cases. In order to prevent the progression of the renal insufficiency in LCAT patients, we have been developing a life-long enzyme replacement therapy via auto-transplantation of preadipocytes manipulated to produce and supply normal LCAT enzymes in plasma. A similar strategy can essentially be applied for the treatment of any kinds of serum enzyme deficiencies. If you are interested in our original therapy, please contact us for details.
Koutaro Yokote, MD, PhD
Email: kyokote (Here at mark) faculty.chiba-u.jp
Masayuki Kuroda, PhD
Email: kurodam (Here at mark) faculty.chiba-u.jp
Phone: +81-43-226-2718
Fax: +81-43-226-2718